XJTU team achieves breakthrough in zinc-based flow batteries
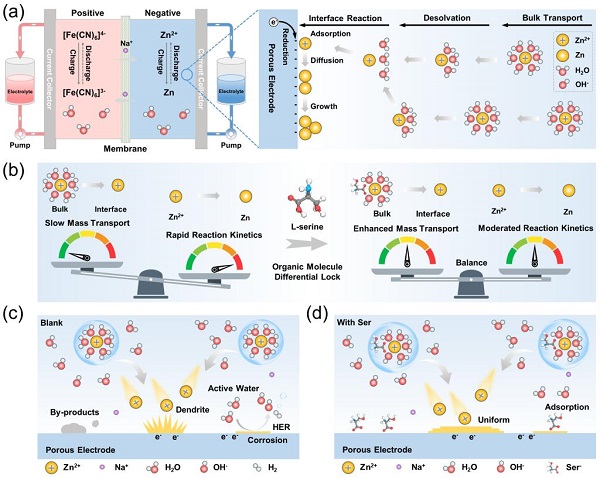
Alkaline zinc-based flow batteries have long been a research hotspot in long-duration energy storage technologies for new power systems, owing to their unique advantages of high safety, high voltage, and low cost.
However, a kinetic mismatch on the anode side between slow zinc-ion transport and rapid electrochemical reactions leads to zinc dendrite growth and irreversible side reactions, which limit the battery's cycle life and commercialization.
Current research has focused on designing anode-side electrolyte additives to alleviate this contradiction by enhancing ion transport rates or regulating reaction kinetics. However, achieving efficient synergy between the two remains a significant challenge.
Therefore, there is an urgent need to develop novel electrolyte additives capable of precisely regulating ion transport and electrochemical reactions to enable high-stability, long-life operation of alkaline zinc-based flow batteries.
In response to the above challenges, a research team led by Academician He Yaling and Professor Li Yinshi from the School of Energy and Power Engineering at Xi'an Jiaotong University (XJTU) proposed the concept of an organic molecular differential lock and a strategy utilizing L-serine (Ser) as an additive to construct such a lock.
The core design is to deploy Ser as a multifunctional additive in the anode electrolyte, establishing a favorable chemical environment that balances zinc-ion transport and electrochemical reactions through phase- and interfacial-partitioned regulation, thereby achieving long-term, stable cycling of alkaline zinc-based flow batteries.
By combining theoretical calculations with experimental characterization, the research team demonstrated the key role of Ser in balancing transport-reaction processes. In the bulk electrolyte, Ser reshapes the zinc-ion solvation structure, enhancing ion transport rates while limiting reduction kinetics.
At the electrode/electrolyte interface, Ser preferentially adsorbs onto the electrode surface, inducing uniform ion flux while slowing down zinc deposition kinetics. Additionally, Ser anchors onto the metal zinc surface to form an interfacial protective layer, suppressing hydrogen evolution and corrosion.
Cycling tests show that batteries containing Ser can operate stably for more than 230 hours at 50 mA•cm⁻² (30 mAh•cm⁻²). This work reveals the multifunctional mechanism of Ser in regulating zinc-ion behavior in both bulk and interfacial phases, providing a new strategy and theoretical basis for developing high-stability, long-life alkaline zinc-based flow batteries.
This achievement was recently published in Advanced Functional Materials under the title Organic Molecular Differential Lock Balancing Transport-Reaction Kinetics for Long-Life Alkaline Zinc-Based Flow Batteries.
-
XJTU and Hong Kong Polytechnic University achieve breakthrough in energy storage dielectrics
December 18,2025
-
XJTU research team reveals North Atlantic Subtropical High's dominance over tropical hydroclimate
December 16,2025
-
XJTU paper wins Cell Press' 2024 Most Popular China Paper Award
December 14,2025

