XJTU's research team proposes new strategy in electrocatalytic carbon dioxide reduction reaction
The F-SAM strategy proposed by the research team can form an efficient triple-phase interface conducive to mass transport and ion/electron transfer.
The electrocatalytic carbon dioxide reduction reaction (CO2RR), which utilizes renewable energy to convert carbon dioxide (CO₂) into high-value chemicals, represents a highly promising strategy. However, beyond the critical role of catalysts, the reaction microenvironment is also a key factor influencing catalytic performance in CO2RR.
Traditional solid-liquid-gas triple-phase interfaces are primarily constructed by spraying polytetrafluoroethylene (PTFE) or other hydrophobic coatings. These methods often result in inhomogeneous interfaces and insufficient ion/electron transport pathways, subsequently leading to reduced selectivity for high-value multi-carbon (C₂⁺) products.
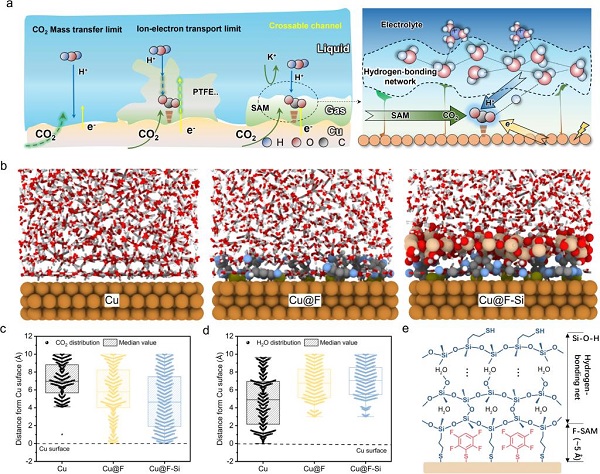
Addressing this challenge, Professor Xiao Chunhui and Researcher Chen Shenghua from Xi'an Jiaotong University (XJTU) proposed a hydrogen-bond network-reconstructed self-assembled fluorinated monolayer (F-SAM) strategy to form an efficient triple-phase interface conducive to mass transport and ion/electron transfer.
This approach involves the co-assembly of F-SAM and siloxane on commercial copper catalysts, denoted as Cu@F-Si. The inner F-SAM layer is uniformly distributed across the electrode surface, creating a molecular barrier that suppresses the hydrogen evolution reaction (HER) while promoting CO₂ transport.
Meanwhile, the outer siloxane hydrogen-bond network regulates the structure of interfacial water molecules, enhancing proton supply to facilitate deep hydrogenation and improve selectivity for C₂⁺ products.
Additionally, the strong interfacial hydrogen-bond network maintains an ideal H⁺/e⁻ transfer pathway, thereby mitigating the detrimental effects of salt deposition and free water on catalyst performance and enhancing the stability of the catalytic layer.
Ultimately, the Cu@F-Si catalyst achieved over 85 percent Faradaic efficiency for C₂⁺ products at a high current density of 502.5 mA cm⁻² and operated stably for over 100 hours at approximately 300 mA cm⁻². This interfacial engineering strategy provides an effective solution for enhancing CO2RR efficiency and holds broad application potential in heterogeneous catalytic systems.
The above research findings were recently published in the prestigious materials science journal Advanced Materials under the title Self-Assembled Monolayer Interface with Reconstructed Hydrogen-Bond Network for Enhanced CO₂ Electroreduction.

